 The European Medicines Agency’s definition of a medication error, published last year, is “an unintended failure in the treatment process that leads to, or has the potential to lead to, harm to the patient”. This, with the addition of a single word, “unintended”, is the definition that Robin Ferner and I suggested 16 years ago. Here I gloss some terms in the definition.
The European Medicines Agency’s definition of a medication error, published last year, is “an unintended failure in the treatment process that leads to, or has the potential to lead to, harm to the patient”. This, with the addition of a single word, “unintended”, is the definition that Robin Ferner and I suggested 16 years ago. Here I gloss some terms in the definition.
• “Failure” signifies that the process has fallen below some attainable benchmark and is the criterion whereby an error is recognised. This derives from James Reason’s definition of an error: “The failure of planned actions to achieve their desired ends without the intervention of some unforeseeable event.”
• The “treatment process” includes manufacture, regulatory controls, commercialisation (labelling, packaging, marketing information), prescribing, transcribing, dispensing, and administration of a drug, and subsequent monitoring and adjustment of therapy in treating symptoms or their causes or investigating or preventing disease or physiological changes. It encompasses not only therapeutic drugs but also oral contraceptives, vaccines, hormones for replacement therapy, radiographic contrast media, and cells.
• The phrase “has the potential” implies that whether or not an error causes harm, it should be logged, for education and future reference, since it may point to measures designed to prevent further similar errors, which may be harmful. This also reminds us of the final clause in Reason’s definition, recognising that errors are often intercepted. For example, a prescriber may write a prescription for “digoxin 250 mg”, but whoever is responsible for administering it may recognise the error and correctly administer 250 micrograms instead, preferably after checking with the prescriber.
• “Unintended” is related to the definition of error in the Oxford English Dictionary: “something incorrectly done through ignorance or inadvertence”. If the failure was intended, that would constitute a violation, a subject for another time.
James Reason classified errors into two broad categories, based on the observation that intentions are carried out according to formulated plans. Either the plan is appropriate but carried out wrongly, resulting in errors that he called slips and lapses, or the plan is inappropriate, leading to errors that he called mistakes. This is potentially confusing, since in common parlance “error” and “mistake” are regarded as synonyms. A more useful approach is Reason’s classification according to whether the error is based on erroneous memory, actions, rules, or knowledge (MARK), as illustrated in the figure.
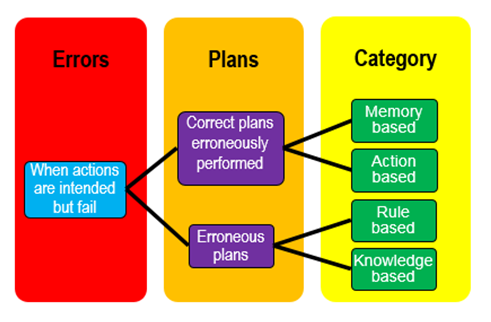
This classification leads to ideas about how medication errors may be prevented, as summarised in the table below. The percentages are taken from a study of methotrexate.

It is fortunate that memory based errors are uncommon, because memory is hard to improve. But an important principle is suggested by the origin of the word “memory”, the Indo-European root [S]MER, which implied being mindful or taking care. In Greek μέρμερος meant fastidious, and memor in Latin meant mindful. To commemorate is to remember someone or something, perhaps with a memorial. A martyr is remembered. To mourn is to remember sorrowfully. A moratorium is a postponement to allow mindfulness, in Sanskrit smṛtyupasthāna. Taking care is a key element in avoiding medication errors.
Jeffrey Aronson is a clinical pharmacologist, working in the Centre for Evidence Based Medicine in Oxford’s Nuffield Department of Primary Care Health Sciences. He is also president emeritus of the British Pharmacological Society.
Competing interests: None declared.