There’s a growing consensus that publishing science through journals is a broken system. But who has the power to change it? Those who fund research have most power, which includes pharmaceutical companies. So far they have not exercised this power, but should they? A meeting in London last week organised by Oxford PharmaGenesis addressed this question.
There’s a growing consensus that publishing science through journals is a broken system. But who has the power to change it? Those who fund research have most power, which includes pharmaceutical companies. So far they have not exercised this power, but should they? A meeting in London last week organised by Oxford PharmaGenesis addressed this question.
The meeting included stakeholders from all the relevant groups: academics, patients, editors and publishers of traditional journals, entrepreneurs producing new ways of publishing science, pharmaceutical companies, regulators, and public funders of research.
I have blogged many times on the failures of the current system, but perhaps the most important failures are that most research is not accessible to many; data are not shared; peer review and editorial decision making are not transparent; papers are usually required to be short; results are scattered through thousands of journals; much of what is published is of low quality yet much that should be published is not published; and the system is slow, inefficient, corrupt, wasteful, and expensive.
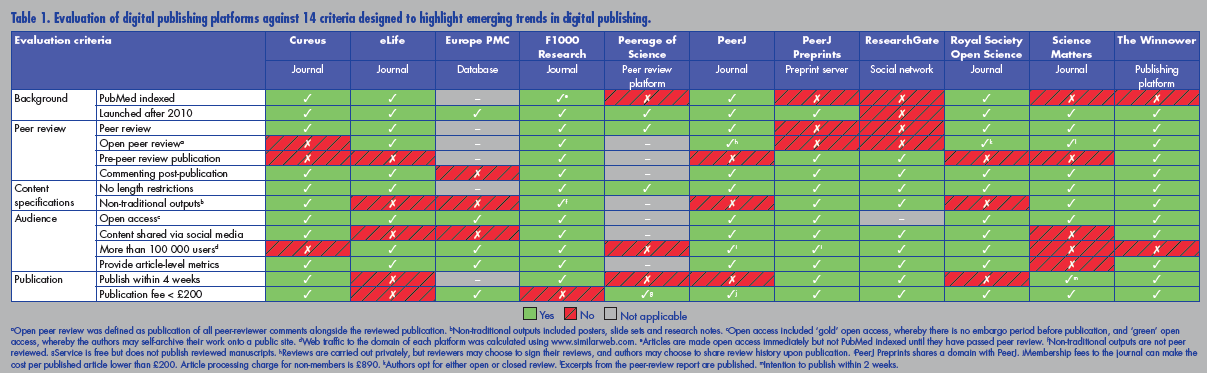
Many entrepreneurs have recognised these failings and are developing ways to overcome them. These developments are happening fast, and the figure below [click to enlarge] summarises some of them. Source: http://www.healthsciencelive.com/UK/987041
The developments include data sharing, open access, fully transparent real time peer review, abolition on restrictions of length, inclusion of video, article level metrics, and extensive links with social media. The use of preprints—whereby studies are shared before submission to a journal and before peer review—is rapidly gathering pace; the system has been used in physics, mathematics, and astronomy for decades but has until recently failed to appeal to authors in biomedicine.
One thing maintains traditional journals and blocks development: that universities and others continue to use journal impact factors (or at least the status of journals) as the prime means for assessing the quality of their researchers. This has long been recognised as unscientific in that there is little or no correlation between the impact factor of journals and the citations of individual articles within the journals—because the impact factor is largely driven by small numbers of articles that are highly cited.
Almost everybody, including researchers, university authorities, and even editors of high impact journals, bemoans the use of impact factors, and it’s long seemed absurd to me that universities should effectively outsource such a core function to an expensive, arbitrary, and corrupt system.
But no individual university or researcher, no matter how eminent, has the power to break the system and allow new ways of publishing science to flourish. One group that does potentially have the capacity is funders of research, and some funders are leading the way.
The Wellcome Trust has been at the forefront, requiring all the research they fund to be open access and creating Wellcome Open Research, which converts the publishing of science from a process controlled by journals, editors, and publishers to a service for researchers. The Gates Foundation earlier this month took the bold step of requiring all the research they fund to be open access immediately—not with the usual delay of six months or more that allows journals to maintain their dominance.
A big proportion of biomedical research, probably more than half, is funded by pharmaceutical companies, but they have largely shied away from using their position to promote innovation in publishing. As the meeting learnt, this is not because they are content with the present system; they have essentially the same complaints as others.
They have too the special circumstance that for their drugs to be licensed they must submit to regulatory authorities every piece of data and every detail of their clinical trials, and increasingly all of that information is publicly available; what appears in journals are scattered fragments of the research, with a perhaps inevitable bias towards the positive. They also have the special circumstance that in order to avoid off label promotion, it has become convention not to discuss research beyond the approved indications for their products outside the safe harbour of peer reviewed journals and academic meetings.
What holds companies back from following other funders of research in using their muscle to encourage new and better ways of publishing science? One constraint is a worry that paying for research to be open access might be viewed by regulators as promotion in an unlicensed indication (which is illegal worldwide), or promotion to patients (which is illegal outside the US, New Zealand, and Brazil) but the meeting, which included regulators, concluded that this was not a serious problem.
A greater problem is that much of the research funded by pharmaceutical companies is conducted by academics who, like all academics, need to publish in high impact journals to advance their careers. Unlike non-industry funders though, pharmaceutical companies are competing with each other to work with leading academics, and current Good Publication Practice guidelines state that manuscript authors should choose the journal, not study sponsors.
The companies are therefore much more nervous than funders such as Wellcome and Gates of requiring researchers to publish in open access journals or to publish in an equivalent to Wellcome Open Research that would be branded with their name. This nervousness probably also has its roots in the prevailing narrative that sees pharmaceutical companies as “bad guys”—despite the fact that, unlike academics, they are required to provide full data and detail of their clinical trials.
The mood of the meeting was that pharmaceutical companies should be bolder and join the movement to try and improve the publishing of science. The next question is how, and that will be the question addressed at future meetings.
Richard Smith was the editor of The BMJ until 2004.
Competing interest: RS helped organise the meeting and chaired it. He was paid for this. He has also been a paid adviser to F1000Research and has a pension from the BMA, which has income from publishing journals. The meeting was funded by Oxford PharmaGenesis with support from GSK Vaccines, Shire, UCB, and the Wellcome Trust.