Investigators should report rare and very rare adverse events in clinical trials: Igho Onakpoya reports why it is important that all events are reported irrespective of their frequency.
 Even though they may not give a signal in any single trial, a meta-analysis could reveal potentially important drug-adverse event associations that might require further verification. Data from a meta-analysis of such rare events can shorten the time to decision making by regulatory agencies and/or drug manufacturers, especially in cases where the reported harms are severe.
Even though they may not give a signal in any single trial, a meta-analysis could reveal potentially important drug-adverse event associations that might require further verification. Data from a meta-analysis of such rare events can shorten the time to decision making by regulatory agencies and/or drug manufacturers, especially in cases where the reported harms are severe.
The benefit-harm profiles of new medicines are usually not fully known at the time of regulatory approval. This is because most trials are powered to detect benefits but not adverse events and also because adverse events are selectively reported or misinterpreted.
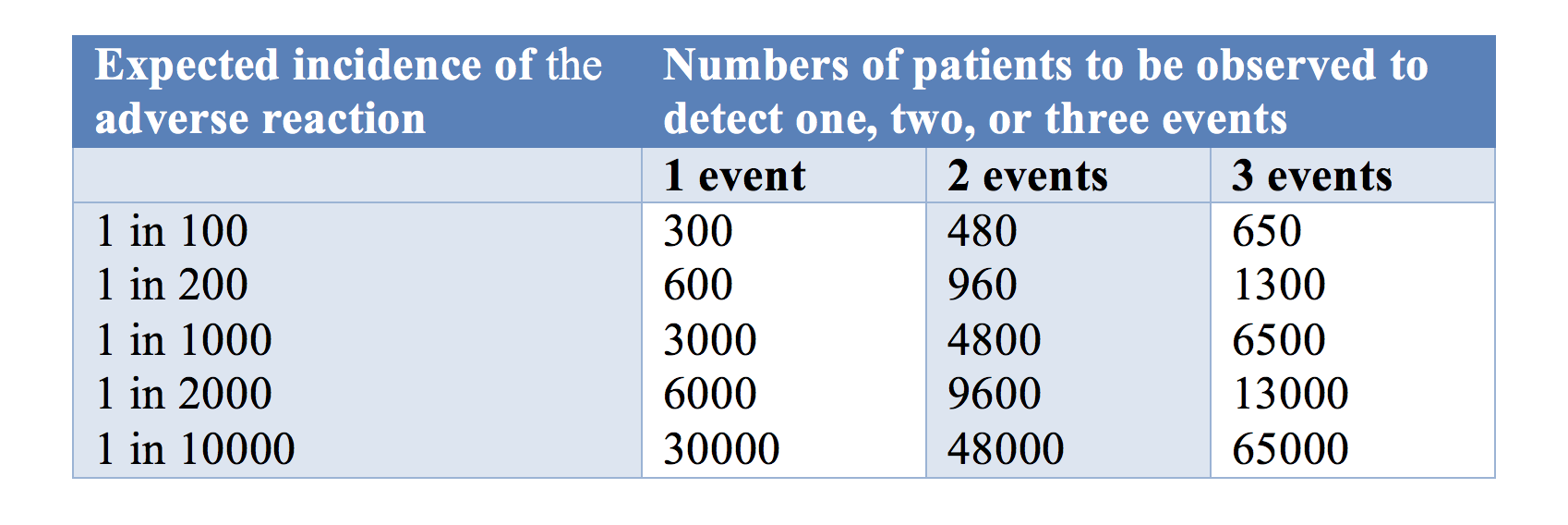
One issue is the number of trial subjects required in a trial to have a 95% chance of detecting an adverse drug reaction at low frequencies is large (Table 1). If a new medication caused serious adverse reactions in 1 in 1000 cases, you need to study 3000 subjects (rule of three) in order to have a 95% chance of detecting even one case.
Table 1: The numbers of patients one would need to observe to have a 95% chance of detecting 1, 2, or 3 cases of an adverse reaction at a given incidence of the reaction
The rule of three means that you need three times as many subjects to observe an event when you assume that the adverse event of interest does not normally occur in the absence of the medication.
Most medicines, however, are marketed on the basis of studies in relatively few subjects. Uncommon or rare events are therefore unlikely to be discovered before marketing. This is why post-marketing surveillance is so important.
Adverse events with frequencies less than 2%, or in some cases less than 5%, are not always reported in clinical trials, even if they are collected, and even though such events could have serious clinical implications.
Several approaches for statistically combining data on rare adverse events have been described in the literature [see here, here and here]. If trial investigators report data on rare and very rare adverse events, the information from several trials can be combined to provide estimates that will help determine whether such events (even though rare in individual trials) warrant further investigation.
Igho J. Onakpoya is a Physician and Research Fellow at the Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford.
Acknowledgements: Jeffrey K. Aronson and Rafael Perera for helpful discussions and input.
Competing interests: None declared