The vaccine “debate” is the art of not talking about what we are talking about […]
Benjamin Mazer: The dog whistle medicine of the anti-vaccine movement

The vaccine “debate” is the art of not talking about what we are talking about […]
What level of evidence should we have before costly, toxic cancer drugs are given to patients? […]
We tend to revere our older colleagues for their clinical wisdom, but this should not compromise patient care […]

Recently, the US Food and Drug Administration (FDA) Commissioner Scott Gottlieb, announced new steps that the agency is taking to enhance transparency of clinical trial information. The FDA is launching a pilot programme whereby selected portions of clinical study reports (CSRs) for nine recently approved new drug applications will be posted on the agency’s website. […]

Although efforts to repeal the ACA in 2017 did not come to fruition, it did not escape unscathed […]

The words we use matter and can influence what we think and how we act […]
We need to find ways to care for all of those at risk, not just those who have the ability to seek care […]

Recently, my home state of California made national headlines when it repealed an HIV criminalisation law and reduced penalties for exposing other people to the virus. It was a landmark decision grounded in science and human rights that will go into effect next month. The law that was repealed had violated people’s fundamental human rights […]
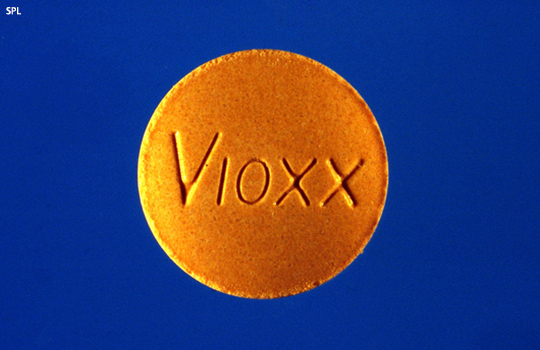
In movie lore, zombies are people who have come back to life to reek havoc and kill innocent people. Now there is a proposal to bring Vioxx (rofecoxib) back to life. The question is whether a resurrected Vioxx will behave like a typical zombie or can it actually help people? As a bit of background, […]

The rise of Trump has ignited fierce debate about the Goldwater rule, but what, if anything, would a formal diagnosis of Trump tell us? […]